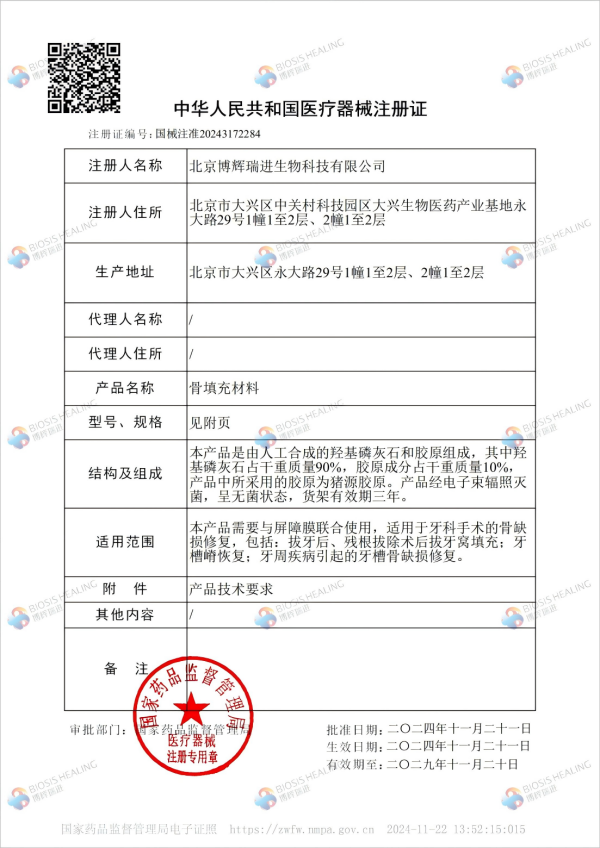
Recently, VIDASIS® SIS/HA Composite Dental Bone Graft Material has been officially approved by the National Medical Products Administration and obtained the registration certificate for Class III medical devices [Registration Number: Guo Xie Zhu Zhun 20243172284].
VIDASIS® SIS/HA Composite Dental Bone Graft Material is composed of a composite of collagen and hydroxyapatite derived from non-crosslinked extracellular matrix material of porcine small intestinal submucosa (SIS material), which is entirely independently developed and patented by Biosis Healing. This product is a bioactive collagen-based Dental Bone Graft Material that must be used in conjunction with a barrier membrane. It is indicated for dental surgical procedures involving bone defect repair, including: alveolar socket filling after tooth extraction or residual root extraction; alveolar ridge restoration; repair of alveolar bone defects caused by periodontal diseases.
VIDASIS® SIS/HA Composite Dental Bone Graft Material is enriched with SIS bioactive collagen, offering advantages such as excellent biocompatibility, enhanced tissue regeneration and repair, and accelerated angiogenesis. It effectively promotes bone regeneration and repair, cellular infiltration, and rapid vascularization, thereby facilitating osseointegration, reducing patient discomfort, and accelerating the healing process.
VIDASIS® SIS/HA Composite Dental Bone Graft Material features a unique elastic scaffold structure that resists deformation or displacement caused by external forces, ensuring enhanced stability of the implanted material. With its superior hydrophilicity and excellent moldability, the product can be easily cut and shaped to match diverse bone defect morphologies, significantly improving clinical efficiency and procedural convenience.

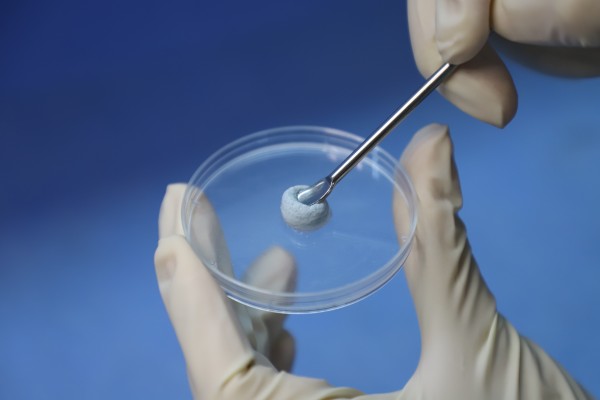
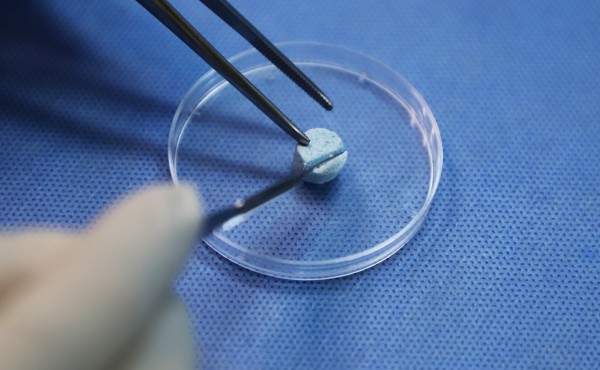
Since VIDASIS® Oral Matrix obtained certification in April 2022 and Biosis Healing officially entered the oral repair field, more than two years have passed. VIDASIS® Oral Matrix have gained recognition and trust from numerous doctors and patients through their excellent product performance, outstanding clinical performance, and absolute cost-effectiveness advantage, gradually establishing brand awareness and reputation in the oral field.
For a long time, the R&D team at Biosis Healing has maintained close communication with oral clinical experts, attentively collecting application feedback from clinical frontline to make refined product adjustments and clarify directions for new product development, striving to provide more effective and convenient product experiences for clinical practice.
VIDASIS® SIS/HA Composite Dental Bone Graft Material underwent rigorous clinical trials and quality testing to ensure clinical safety and effectiveness. This new product certification will further enhance Biosis Healing's product portfolio in the field of oral rehabilitation. We firmly believe that the optimal combination of VIDASIS® Dental Guided Tissue Regeneration Membrane, VIDASIS® Oral Matrix and VIDASIS® SIS/HA Composite Dental Bone Graft Material, will provide dental professionals with more comprehensive and reliable solutions, offering patients improved therapeutic outcomes and cost-effective treatment options to restore oral health and pioneer new chapters in dental care.











版权所有®2019BIOSISHEALING